In the video I showed you both liquid and solid oils. And I said that they are made up of the same compounds. But how can that be? How can they be the same with one being very liquid and the other very solid?
Short answer, they are made up of the same types of carbon chains called fatty acids. However, there are hundreds of different fatty acids, so that is why the oils can vary so much, solid to liquid and more. The acid part of fatty acid is the carboxyl group, COOH, on one end of a carbon chain which, falls within the chemical definition of acidic. And they are fatty and oily, because of the carbon chain tail. About ninety eight percent or more of the oil, either liquid or solid, is made up of these chemical compounds, oils are primarily fatty acids.
So to understand oils you need to understand fatty acids. What is amazing is that there are hundreds of different kinds of fatty acids, whose chains of carbon atoms make up the lipid oils. Every one of these chains of carbon, no matter what is going on within the chain has the same two ends, the carboxyl group and the methyl end. Here is a basic fatty acid where you can see each part :
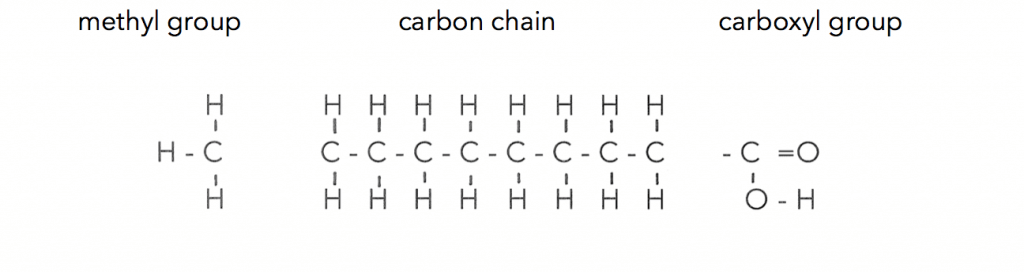
These chains of carbon can be long or short, they can twist and turn, they can bend or kink. The carbon atoms of the chain can be fully attached to hydrogen atoms or partially bonded with hydrogen that creates the wide range of variations. So let’s look at some of the variations that make our solid and liquid oils.
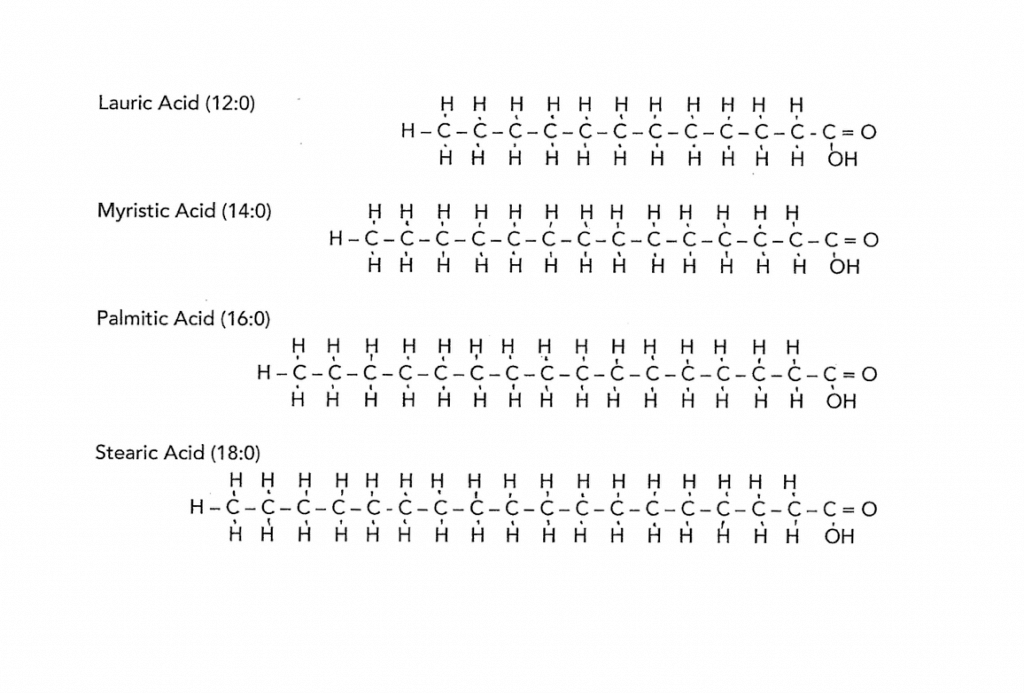
The solid oils are solid because the carbon chains have hydrogen attached all along the length of the chain – this causes the chain to be straight. And like a fist full of pencils these straight chains of carbon lie closely together and appear solid at room temperature. The longer the chains the stiffer they make the oil. To experience this, feel the difference between coconut oil and cocoa butter. The drawing shows four different saturated fatty acids. These are very common ones found in popular oils. Notice they are straight and vary in length.
The liquid oils are made up primarily of similar carbon chains but are missing two, four, six or more hydrogen atoms. Where they are missing these hydrogen atoms the chain bends. If it is missing six hydrogen paired atoms it bends more than missing just two.
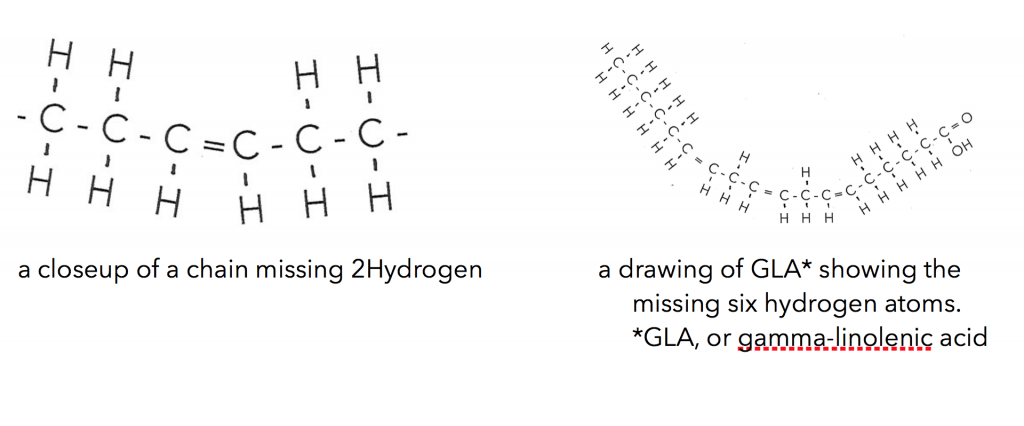
So you can see that understanding the oils both solid and liquid you’ll want to get grounded in the fatty acid types and make up of these oils. These are a few points to get you started:
Oils are made up of many different types of fatty acids.
Solid oils are made up of predominantly saturated fatty acids.
Conversely, liquid oils are made up of predominantly unsaturated fatty acids.
But both saturated and unsaturated fatty acids are found in ALL oils.
And fatty acids don’t exist on their own but are organized into molecules such as triglycerides, phospholipids and cholesterol in the body.


Love the chemistry. Am planning on sharing with my 16 yo daughter as she goes into chemistry class next year and is also very into skin care nowadays.
Thank you.
Each time I see a video or read a paragraph about the chemical composition and the chains one more little light bulb goes on in the long chain in my brain! Thanks, Susan!